Abstract
There remains uncertainty regarding the optimal treatment for mixed phenotype acute leukemia (MPAL), an uncommon leukemia that represents ~3% of de novo acuteleukemia. Because of the dearth of clinical trials, varying approaches are employed: acute lymphoblastic leukemia (ALL) regimens as well as more intensive acute myeloid leukemia (AML) and hybrid therapies. The evolution in the prevailing diagnostic criteria from the EGIL classification to the more narrowly defined WHO2008 and WHO2016 criteria has made the existing literature difficult to interpret. Increasing insight into the molecular heterogeneity of MPAL adds further complexity. Recently published studies (retrospective clinical case series), however, are beginning to suggest that ALL therapy may be the most appropriate form of initial therapy for MPAL.
To further assess the efficacy of ALL therapy for pediatric MPAL, we reviewed the Children's Oncology Group (COG) experience. Possible MPAL cases were identified through the ALL biology studies (both AALL03B1 and AALL08B1 were open to MPAL) and from AAML0531, the phase 3 trial for de novo AML (patients enrolled on this trial subsequently found to have MPAL were taken off study). All identified cases were reviewed centrally. In cases where the original flow cytometry testing was inadequate to establish the diagnosis, flow cytometry was repeated with an expanded antibody panel using banked diagnostic specimens. Only cases meeting WHO2016 criteria were retained. Patients were treated per physician discretion and not on a therapeutic clinical trial. Supplemental data on treatment and outcomes were collected.
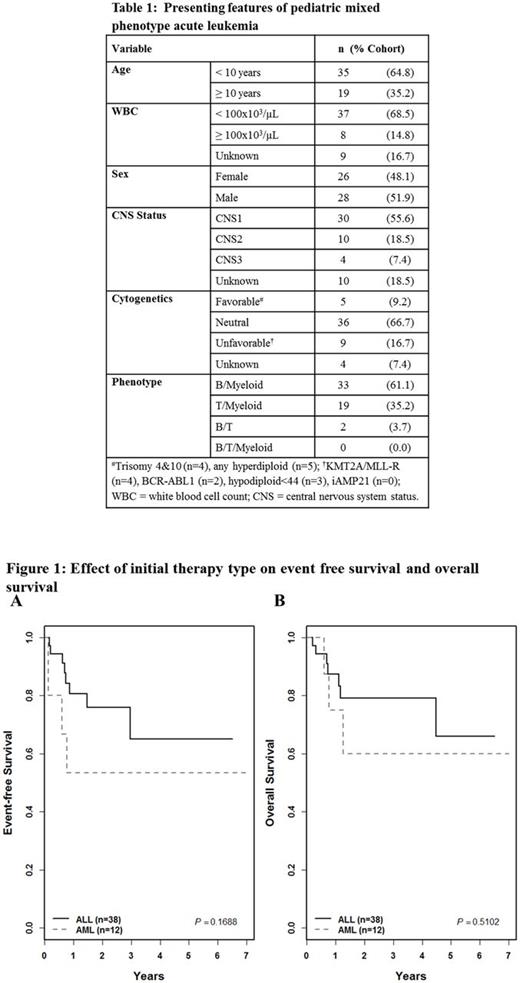
From the study databases, 97 potential MPAL cases were identified; of these, 54 cases (56%) were confirmed to be MPAL via central review. Patients were diagnosed between 2003 and 2016. The majority of patients were <10 years of age and B/Myeloid MPAL (B/My) was the most common phenotype. Fewer than a third of patients presented with hyperleukocytosis, adverse recurrent cytogenetics, or central nervous system involvement (Table 1). In the cohort, 38 patients (70%) received ALL induction therapy, 12 (22%) AML induction, and 3 hybrid induction; 14 (28%) patients received hematopoietic stem cell transplantation (HSCT). The CR rate was 71.1% [27/38], 66.7% (8/12) and 100% (3/3) in patients receiving ALL, AML and hybrid induction, respectively (p=0.761). CR was achieved in 78.8% of B/My cases [26/33], 68.4% T/My cases [13/19], and no B/T cases (0/2, p=0.074). Treatment became more heterogeneous further into therapy due to varying incorporation of ALL, AML and hybrid elements and the varying use of HSCT. Three-year EFS was 65±17% in patients started on ALL therapy and 53±26% in patients started on AML therapy (p=0.169, Fig. 1A); 3-year OS was 79±15% and 60±27% respectively (p=0.510, Fig. 1B). SCT was not associated with a difference in EFS (p=0.935) or OS (p=0.726). In 20 patients receiving ALL therapy alone without SCT, 3-year EFS was 59±22% and OS 79±18%.
This series represents a large cohort of centrally-reviewed pediatric MPAL cases diagnosed according to the most recent WHO2016 criteria and treated with COG-style chemotherapy. Our results add to the growing body of literature suggesting that ALL regimens may be effective for pediatric MPAL. It also suggests, however, that induction failure and relapse are still relatively common with ALL therapy and that alternative forms of treatment are necessary for some. The limited size of the sample and its heterogeneity preclude any conclusions regarding the relative efficacy of AML therapy and role for HSCT. Given the greater morbidity and mortality associated with these forms of therapy, consideration should be given to reserving their use for patients with a suboptimal response to ALL therapy. Continued variability in accurately diagnosing and treating MPAL as seen here also underscores the necessity of well-delineated clinical trials with integrated central review to advance MPAL therapy. Our results therefore further support the basis for the first-ever prospective evaluation of high risk ALL therapy for pediatric MPAL in a trial now being planned within the COG. In this trial, minimal residual disease testing with multiparameter flow cytometry will be employed to identify patients responding well to ALL therapy versus those who may benefit from more intensive AML therapy and transplantation.
Mullighan: Loxo Oncology: Research Funding; Amgen: Consultancy. Hunger: Erytech Pharmaceuticals: Consultancy; Novartis: Consultancy; Jazz Pharmaceuticals: Honoraria; Amgen: Consultancy, Equity Ownership. Berman: AGADA Therapeutics: Research Funding. Zweidler-McKay: ImmunoGen: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal